Abstract
Introduction: Immunochemotherapy is standard of care treatment for previously untreated patients (pts) with advanced stage follicular lymphoma (FL). However, the majority of pts relapse, with around 20% relapsing within 2 years. Obinutuzumab (GA101; G) is a glycoengineered type II anti-CD20 monoclonal antibody (mAb) with increased antibody-dependent cell-mediated phagocytosis and cytotoxicity, and direct B-cell killing, compared with the type I mAb rituximab (R). The randomized Phase III GALLIUM study (NCT01332968) compared the efficacy and safety of G-chemotherapy (G-chemo) vs R-chemotherapy (R-chemo) in previously untreated pts with advanced stage FL. In the primary analysis (PA), a significant improvement in the primary study endpoint of investigator (INV)-assessed progression-free survival (PFS) was demonstrated with G-chemo relative to R-chemo after 34.5 months' median follow-up. Results for other time-to-event outcomes (including time-to-next treatment; TTNT) were supportive, and additional analyses demonstrated a higher rate of minimal residual disease, and a lower risk of disease progression within 24 months, in the G-chemo arm. Here we report the results from an updated analysis of time-to-event endpoints and safety in the GALLIUM study.
Methods: In GALLIUM, enrolled pts were aged ≥18 years with previously untreated FL (grades 1-3a), Stage III/IV disease (or Stage II with bulky disease), and ECOG PS 0-2, and requiring treatment according to GELF criteria. Pts were randomized 1:1 to receive G 1000mg IV (days 1, 8 and 15 of cycle 1 and day 1 of subsequent cycles) or R 375mg/m2 IV (day 1 of each cycle) plus chemotherapy for 6 or 8 cycles, depending on the chemotherapy backbone (CHOP, CVP, or bendamustine). Chemotherapy backbone was a stratification factor and was chosen upfront by the participating centers. Pts with a complete or partial response at the end of induction received maintenance with the same antibody every 2 months for 2 years. The data cut-off for the current analysis was February 12, 2018.
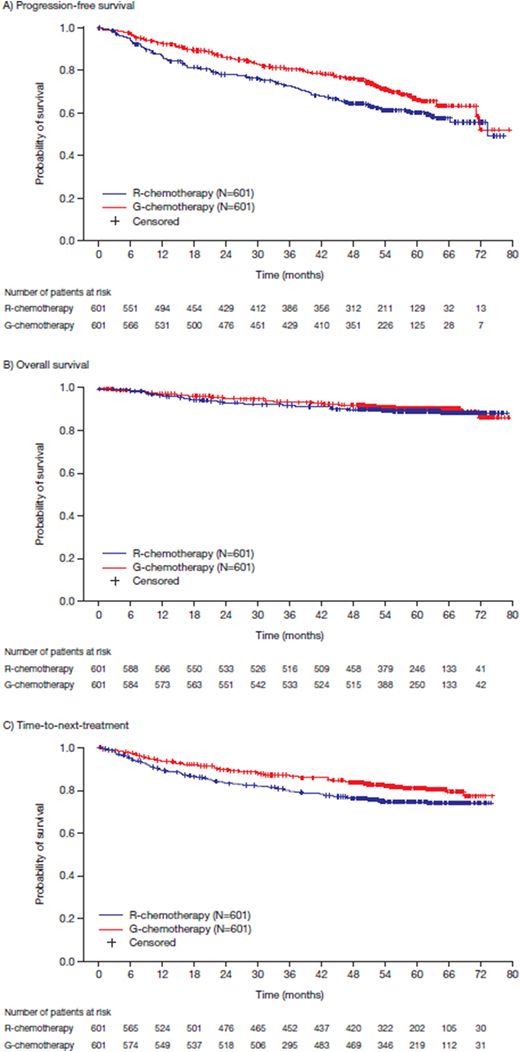
Results: In total, 1202 pts with FL were enrolled (G-chemo, n=601; R-chemo, n=601), with a median age of 59 years (46.8% male). After 57.3 months' median follow-up, a sustained and clinically meaningful improvement in INV-assessed PFS was observed with G-chemo relative to R-chemo (HR 0.73; 95% CI: 0.59, 0.90; p=0.0034; 4-year PFS rate: G-chemo, 78.1% [95% CI: 74.4%, 81.3%]; R-chemo, 67.2% [95% CI: 63.1%, 71.0%]; Figure 1A). Since the PA, 114 new PFS events (57 in G-chemo and 57 in R-chemo) had occurred. Overall survival (OS) data, which remain immature, were comparable across treatment arms (HR 0.88; 95% CI: 0.61, 1.27; p=0.49; 4-year OS rate: G-chemo, 92.6% [95% CI: 90.1%, 94.4%]; R-chemo, 90.3% [95% CI: 87.6%, 92.5%]; Figure 1B). A sustained benefit in favor of G-chemo was observed in TTNT (HR 0.70; 95% CI: 0.54, 0.90; p=0.0046; 4-year TTNT rate: G-chemo, 84.2% [95% CI: 80.9%, 86.9%]; R-chemo, 76.7% [95% CI: 73.1%, 80.0%]; Figure 1C). Since the PA, 29 and 36 additional pts in the G- and R-chemo arms, respectively, had received new anti-lymphoma treatment. At cut-off, 54 (9.1%) pts in the G-chemo arm and 61 (10.2%) in the R-chemo arm had died. Common causes were disease progression (G-chemo, n=21 [3.5%]; R-chemo, n=28 [4.7%]) and AEs (G-chemo, n=24 [4.0%]; R-chemo, n=24 [4.0%]). The most common fatal AEs were infections (9 vs 4 pts, respectively) and new malignancies (5 vs 6 pts, respectively). Incidences of AEs of any grade were comparable across the G-chemo (99.8%) and R-chemo (99.5%) arms, although grade 3-5 AEs (79.2% vs 71.2%) and serious AEs (48.7% vs 42.2%) were more common in the G-chemo arm. AEs led to discontinuation in 16.3% of G-chemo pts and 14.6% of R-chemo pts. As in the PA, the incidence of grade 3-5 infections (22.2% vs 18.6%), grade 3-5 neutropenia (and associated complications reported as AEs, 48.4% vs 41.4 %), and grade 3-5 second malignancies (6.9% vs 4.4%) was numerically higher in pts receiving G-chemo than R-chemo.
Conclusions: In line with the PA, the results from this updated analysis of the GALLIUM study, with a median follow-up of almost 5 years, reinforce that G-chemo provides clinically meaningful improvements in outcomes relative to R-chemo in previously untreated FL pts. OS data remain immature, with additional follow-up needed to draw conclusions. Safety data are consistent with those reported in the PA.
Townsend:Gilead: Consultancy; Roche: Consultancy. Buske:Roche: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Bayer: Research Funding. Cartron:Roche: Consultancy, Honoraria; Sanofi: Honoraria; Gilead Sciences: Honoraria; Janssen: Honoraria; Celgene: Consultancy, Honoraria. Cunningham:Roche pharmaceuticals: Research Funding. Dyer:Roche: Honoraria, Research Funding. Gribben:Medical Research Council: Research Funding; Acerta Pharma: Honoraria, Research Funding; Roche: Honoraria; Novartis: Honoraria; Janssen: Honoraria, Research Funding; TG Therapeutics: Honoraria; Abbvie: Honoraria; Wellcome Trust: Research Funding; Unum: Equity Ownership; Pharmacyclics: Honoraria; NIH: Research Funding; Cancer Research UK: Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Kite: Honoraria. Hess:Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Honoraria, Other: travel expenses, Research Funding; CTI: Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Keller:BMS: Consultancy; Celgene: Research Funding; MSD: Consultancy; Takeda: Consultancy, Research Funding; Janssen-Cilag: Consultancy, Equity Ownership; Roche: Consultancy. Kneba:Roche: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria. Malladi:Roche: Membership on an entity's Board of Directors or advisory committees. Neidhart:Gilead: Consultancy; Roche: Consultancy, Other: Travel support and lecture fees. Rusconi:Celgene: Research Funding. Zhu:Beijing Cancer Hospital (Peking University Cancer Hospital): Employment. Catalani:Roche: Employment. Knapp:Roche: Employment. Zeuner:F. Hoffman-La Roche: Employment, Equity Ownership. Hiddemann:Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; F. Hoffman-La Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer: Consultancy, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Marcus:Roche: Consultancy, Other: Travel support and lecture fees ; Gilead: Consultancy; F. Hoffman-La Roche: Other: Travel support and lecture fees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal